by Brian Theyel, MD PhD
Summary:
• Currently available data indicate that both the Pfizer and Moderna vaccines are safe and effective.
• The protective effects of both vaccines likely provide significant benefit starting around 6 days after the first injection, with maximal benefit by 7-14 days after the second dose.
• The favorable risk/benefit ratio of the vaccines and lethality of Covid-19 make a strong case to get the vaccine, particularly for those who will be “leading the way” on this.
Dear Reader,
I hope this finds you and yours safe and well as we exit the strangest of holiday seasons. I wanted to share a few of my thoughts and excitement about the two available vaccines for Covid-19. A frustration that I have had when listening to news summaries about these vaccines is that they rarely tell you just how quickly the vaccines start to work. This is, of course, a highly relevant piece of information for those of us who have been lucky enough to have early access. The scientist in me said “show me the data!” A quick google search brought me to the FDA summaries of evidence for both vaccines, where I found overwhelmingly reassuring data about the safety of both vaccines, as well as two powerful graphs:
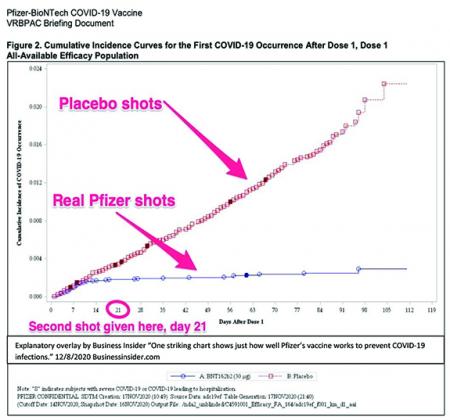
Explanatory overlay by Business Insider “One striking chart shows just how well Pfizer’s vaccine works to prevent COVID-19 infections.” 12/8/2020 Businessinsider.com
I think these graphs really bring home just how effective the vaccines are and shed some light on when the vaccines start yielding some benefit. The first graph is from the Pfizer-BioNTech Phase III trial. In red you will see data points indicating what proportion of patients in the placebo group had symptomatic Covid-19 a certain number of days out from their first injection. In blue you will see the proportion of individuals who were in the vaccine group who had symptomatic Covid-19. Note the dramatic divergence in the curves beginning around 11 days after the first dose was administered. Taking into account that the average incubation time for SARS-CoV-2, the virus that causes the disease Covid-19, is about 5-6 days, it looks probable that the vaccine may start to yield some benefit by 6 in terms of preventing symptomatic disease from taking hold after exposure.
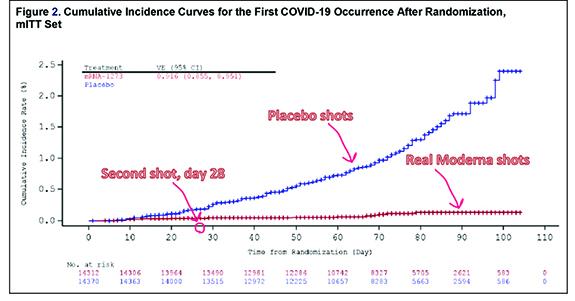
The Moderna data appear to suffer from a relatively low rate of infection early on in the study, but if you look closely the two lines (in this case the placebo group is in blue and the vaccine group is in red) start to diverge at around 11-15 days before widely diverging after day 22. For both the Moderna and Pfizer-BioNTech vaccines these data suggest a preventive benefit beginning somewhere between 6-11 days after the first shot. A closer look at the numbers in both FDA summary documents shows that this benefit is somewhere between about 50-80% early on, before rising to about 94% for both vaccines by Day 28 for the Pfizer vaccine and by Day 42 for the Moderna vaccine.
A critical point remains: we know that the vaccines work extremely well at preventing someone from developing symptoms of Covid-19. What we do not know is whether, and how well, the vaccines prevent transmission. A virus, after all, can theoretically still be shed while vaccinated individuals are fighting it off. Therefore, it is critical - and likely necessary for us to beat this virus quickly – to keep wearing masks, ventilating our buildings, washing our hands and limiting our social contact at least to the extent that we have been.
In my estimation, these data are highly compelling. There is an excellent safety profile for both vaccines: fatigue, headaches, muscle aches, chills/fever are common but manageable, and around 11 significant allergic reactions – all successfully treated - after over two million administered doses. This suggests that for most the benefits of getting these important vaccines will likely outweigh the risks. Getting the vaccine is an individual choice, and I would encourage any who are on the fence to discuss it with your doctor.
My own decision process went something like this:
When I became a physician, I swore the following: “I will prevent disease whenever I can, for prevention is preferable to cure. I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” When it comes to deciding whether to take a vaccine that has the potential to save millions of lives, including patients, friends, and family, and is dramatically less risky than driving my car, the decision was a “no-brainer.” I will be receiving my second dose of the Pfizer vaccine on January 8th and boy do I feel good about the fact that this vaccine, alongside our hospital’s excellent implementation of diligent mask wearing, enhanced building ventilation, and hand-washing, will protect me, my family, my community, and the patients I see in the Psychiatric Emergency Room I work in.
My best wishes to you and yours for a happy, and healthy, new year.
-Brian Theyel, MD PhD
Acknowledgements: While I am ultimately responsible for the content of this document, I would like to acknowledge the valuable edits and input from the following trusted (and treasured) colleagues and family: Tracey Guthrie, MD; Al Olivares, MD; Laura Whiteley, MD; Barry Connors, PhD; Mary Hohenhaus, MD; Paul Theyel, MS; Patricia Bero, MPS; Elizabeth Sullivan, MD; Raymond Powrie, MD.
References:
1. Pfizer-BioNTech COVID-19 Vaccine VRBPAC Briefing Document. Dec 10th, 2020. Food Drug Administration document.
2. Moderna COVID-19 Vaccine VRBPAC Briefing Document. Dec 17th, 2020. Food Drug Administration document.